4 Steps to Improve Your Reactive Dyeing with pH Monitoring
For companies dependent on consistent coloration of their products, pH can play a major factor in their success. My colleague, James, spoke about pH sensitivity in a prior blog. He explains, “A changed shade (due to pH) can affect consistency, brand awareness, and may cause the customer to think the product is bad.” Simple yet vital, an understanding of how different materials, dyes, and chemicals will react at different pH levels is important for creating a consistent final product.

But first, we must understand: What are reactive dyes?
Just as the name indicates, these dye molecules react with the cellulosic fiber and create a covalent bond. This covalent bond allows the dye molecules to become one with the fiber, becoming almost impossible to wash away or rinse out. They share electrons at the atomic level, making them dependent on each other. This results in high colorfastness and wetfastness, if applied correctly.
So, how do we maintain the right pH throughout our reactive dyeing process?
1. Set the Best Dye Bath (Liquor)
First we must set our dye bath, or as coined in the industry, the liquor. You want your pH to be neutral to slightly acidic. If pH is too high (alkaline), there could be premature hydrolysis. This means the reactive dye can strike too soon, which creates an uneven dyeing and inconsistent shade variations from run to run.
2. Choose the Appropriate Exhaustion Method
There are several different exhaustion dyeing methods, but it’s best for every customer to run trials in their lab to find out which method works for them. Each method has its own distinctive advantages, these are standard features of each:
- Standard Method – Temperature rise for normal cellulosic fibers
- Standard Method – Isothermal at 60°C for normal cellulosic fibers
- Specific Method – Migration for mercerized cotton, viscose rayon, tightly wound packaged and high twist yarns
- Specific Method – Isothermal at 80°C for mercerized cotton, viscose rayon, tightly wound packaged and high twist yarns.
3. Perform a Progressive Dosing of Alkali
Once you determine which exhaustion method works for you, I recommend a progressive dosing method of alkali for the pH gradient. This allows for an even strike of the reactive dyes, giving the fiber a level dyeing. Once you reach your desired temperature, you should apply your alkali as follows:
- 1/10 of total g/l of alkali; hold 7 to 10 minutes
- 3/10 of total g/l of alkali; hold 7 to 10 minutes
- 6/10 of total g/l of alkali; depending on depth of shade, run time will be 30 – 60 minutes at temperature.
4. Use a Soaping Process to Rid of the Alkali
Once dyeing is complete, the alkali is still present with the cellulosic fibers and must pass through a soaping process. Here is when we’ll give the fibers a good rinse to rid of the alkali. We need to neutralize the alkali with many warm to hot rinses or an adequate amount of acid.
Below are my suggestion for soaping, but keep in mind you may choose to alter the number of rinses based upon yours – and your customer’s – quality requirements.
Neutralization Without Acid
- 1 cold rinse, hold for 5 minutes
- 3 rinses at 48°C, hold for 7 – 10 minutes each
- 1 hot rinse at 82 – 93°C, hold for 10 minutes (time and temperature may vary, depending on depth of shade and amount of alkali used during dye process)
Neutralization With Acid
- 1 cold rinse, hold for 5 minutes
- 3 rinses at 48°C, hold for 7 – 10 minutes each
- Add 0.50% acid. Then heat to 82 – 93°C and hold for 10 minutes (time, temperature, and amount of acid may vary, depending on depth of shade and amount of alkali used during dye process)
How pH Variability Affects Absorption
When a pH is too low, there’s only a partial reaction of the dyes to the fiber. You’ll see less absorption leading to lighter and inconsistent shade matching. The result is poor color quality, colorfastness, washfastness, and crocking (wet and dry).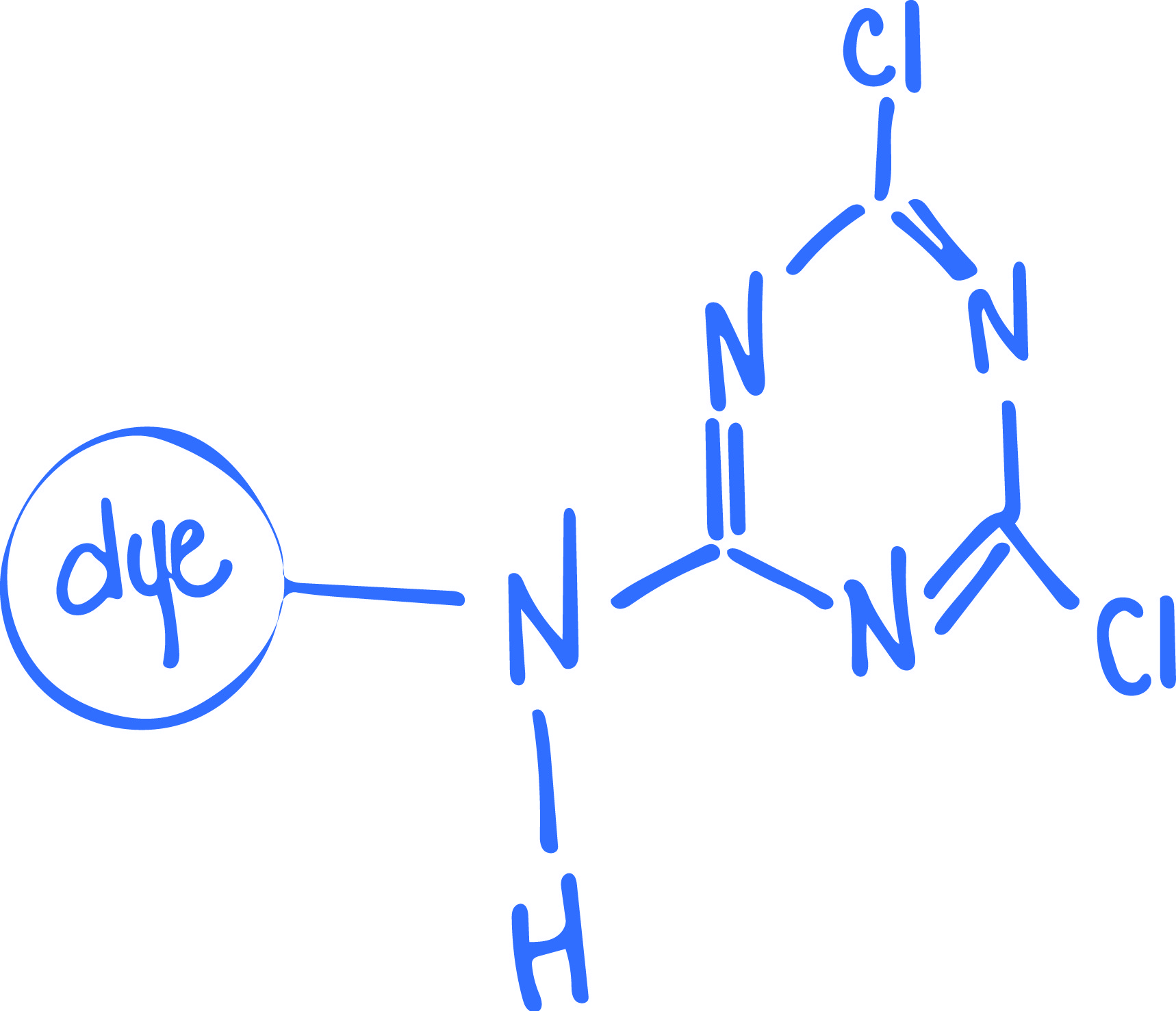
Too high a pH will create a hydrolysis of the dye in the water, leading to almost no absorption to the fiber. This has the same low quality color and fastness results mentioned before, but now at a lower pH.
Of course, the amount of alkali is determined by other factors in the process. These include the depth of shade needed, type of cellulosic fiber, and the type of reactive dyes used. You should consider all of them.
Depending on the depth of shade, you may include other additives. For lighter to medium shades, you may choose to use soda ash only. For medium to dark shades, you may need to add a small amount of caustic soda.
Even and level dying relies on monitoring pH throughout the reactive dye process. From liquor set up to the neutralization during the soaping, monitoring each step along the way can guarantee good fastness results. Not too high and not too low, and the results lead to the reactive dyes becoming part of the fiber. This is the best way to promote the longevity of color in the end use garment.



