Why Do Dyes Fade? A Look at Low Lightfast Dyes
When we consider the question, What causes dyes to fade?, we’re figuring out what dyes are best to use in what situations. Why is understanding fading important? If you choose a dye with poor light fastness, or one that fades quickly, you risk color variation.
For example, if you dye fabric for outdoor clothing, the sunlight will damage the color over time. It will fade even faster if you’re using a dye with poor light fastness. Your end customer will not be a happy camper. When choosing dyes, we need to fit them for their end use. This includes their ability to withstand light if needed. We want happy campers!
Understanding the Structure of Dyes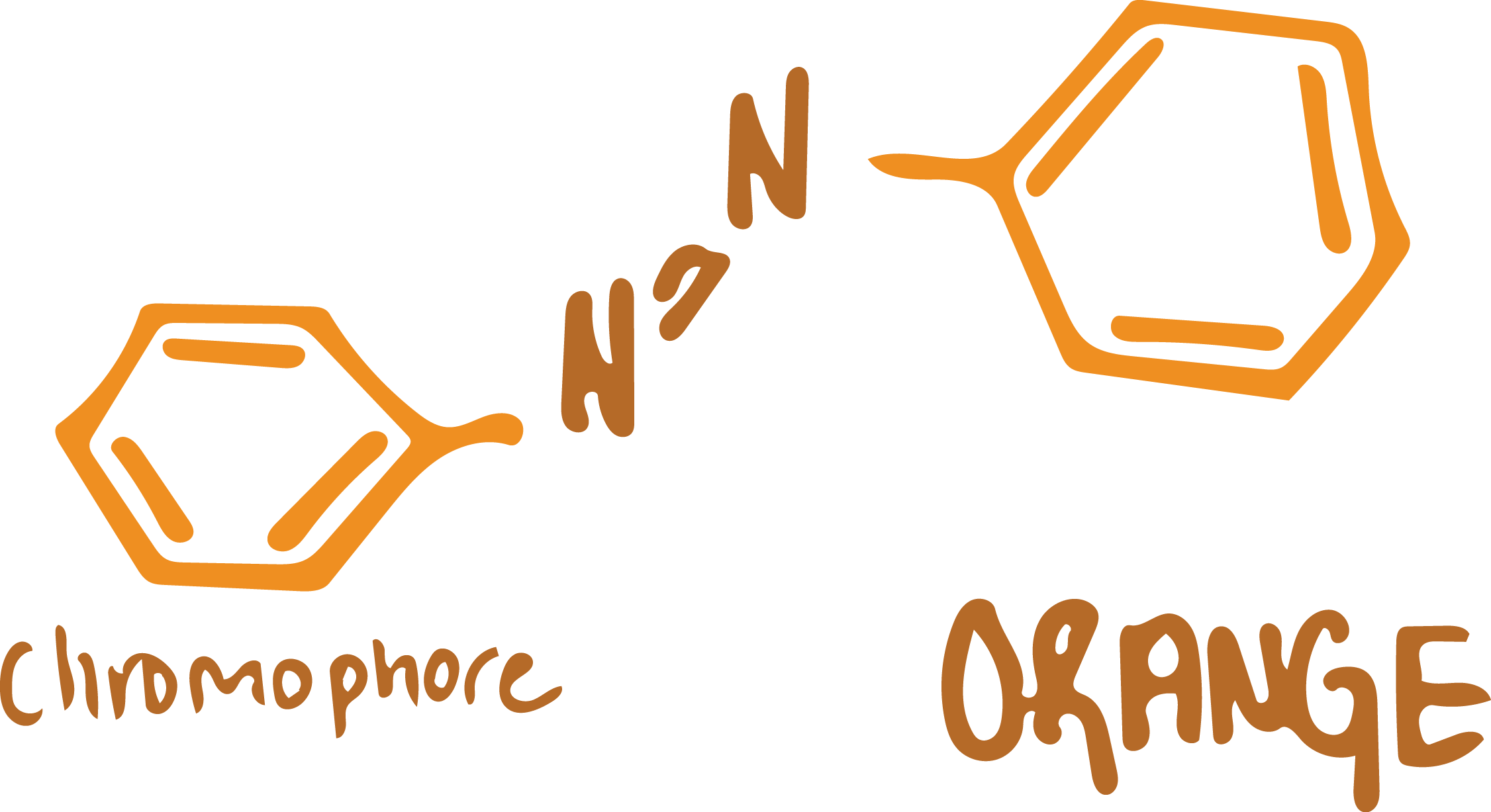
So, why do dyes fade? To answer this, we need to review the characteristics of dyes that provide color.
Simply put, dyes are organic compounds that absorb light in the visible spectrum. These compounds have a color bearing group, called a chromophore, that provides the hue. They’re structured with a conjugated system, which has alternating double and single bonds. They also exhibit resonance of electrons. If any one of these characteristics is not in the molecular structure, we’ll lose the color (1).
This point is key to understanding why color fades. I am going to explain using azo dyes as an example, which comprise the largest group of dyes made commercially in today’s world.
Three Factors that Effect Colorfastness
It is a well-known fact that sunlight causes colors to fade. It is a less known fact that certain chemicals and pH can have the same effect. Let’s explore what happens in all three instances.
The Damaging Effects of Sunlight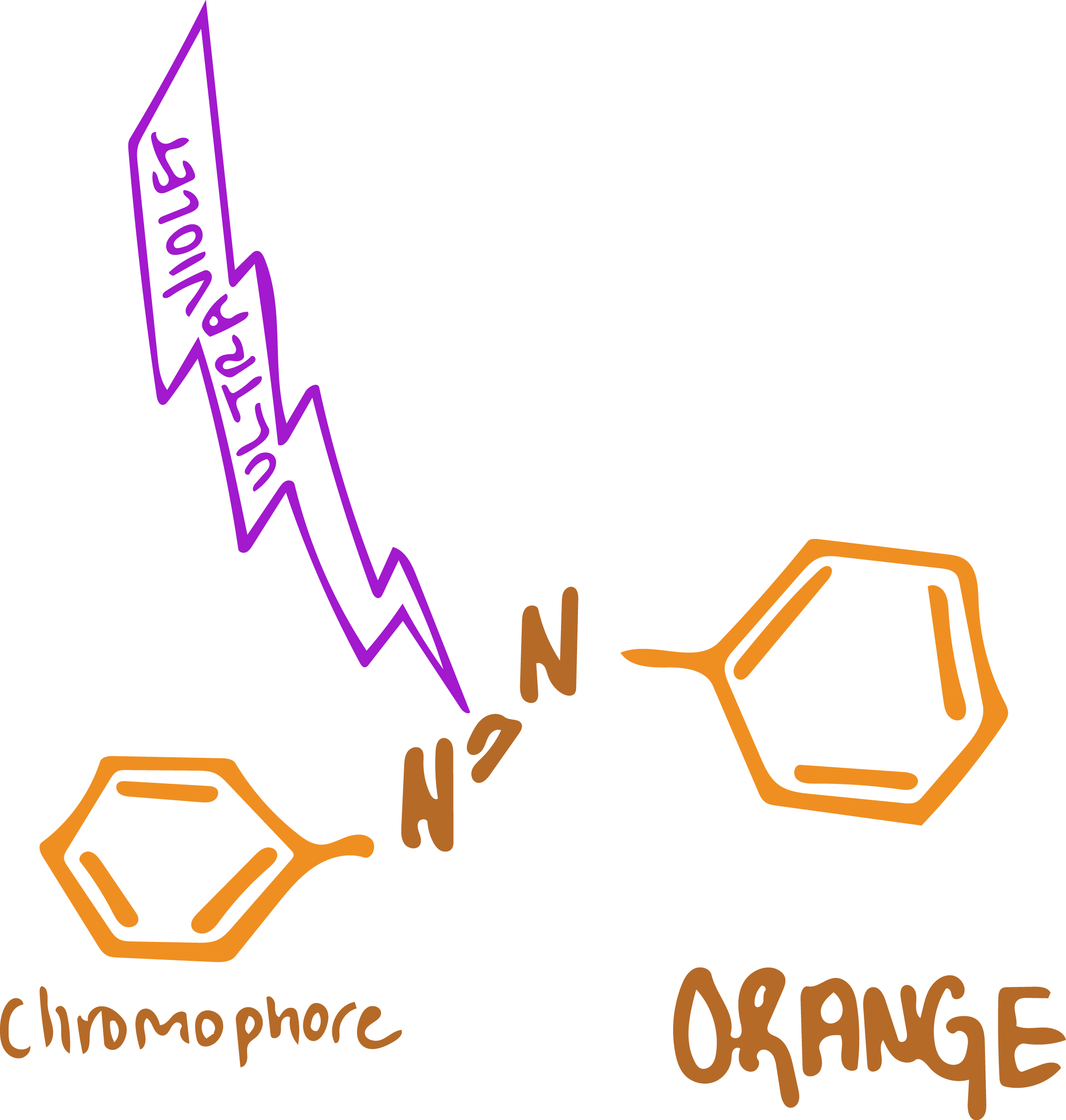
When dyes are exposed to sunlight, the ultraviolet light breaks the azo bond in the dye molecule. This can happen over a short or a long period of time depending on the structure of the dye. It is a natural process that is difficult to avoid. Even the most lightfast of dyes will eventually fade or change color.
pH Level Variability
In the same manner a very high or very low pH can destroy the azo bond. In some cases, this can happen fast…even less than a minute. I have seen this happen in the lab many times. One second the blue color is there, and in another second it is gone.
Chemical Usage Effects
We know what happens when we spill bleach on our favorite sweater or shirt. The color fades and vanishes. Now, it’s a chemical causing the rupture of the azo bond in the dye molecule.
This reaction is also used in our environment to clean water. Introducing bleach to a colored waste water system is an effective method of getting rid of the color. Over time, with repeat injections of bleach a waste water treatment plant can use this in the initial phase of their treatment program.
Should I not use fading dyes?
Thus far, I may have given you the impression dyes that fade are bad dyes. But, this is not necessarily true. Here are examples of when it is a good thing.
When driving through construction areas, you may notice a graded green mat on sides of the roads. This is hydro mulch. It contains a proprietary mix of products that contains colored straw and grass seed. They dye the mix green and it fades over time as the grass begins to germinate and grow. By the time the grass is a couple of inches tall, the green color fades and you’re left with the natural grass color.
Another use for fading dye is in marking painted ceiling areas. These products are useful when you want to track where you’re painting a ceiling white. Using a tinted red paint that turns white as it dries will allow for an even coat, since you can track where you’ve already applied the paint.
Similarly, when sealing concrete, it is sometimes hard to see where you applied sealant. Some opt to use a sealant that is tinted green and fades to clear when dry. This is another example of where there’s value in using fading dyes.
The Curious Case of Red Dyes
Why does it seem that red dyes fade faster than other dyes? There are many theories about the cause, but none are definitive. I believe it is a combination of the size of the red dye molecule, the chromophore, and the structure of the dye. The characteristics of these three things produce the perfect storm for the color red to absorb a lot of energy in shorter wavelengths of light. This makes it weak over time and causes the color to fade faster.
Keep in mind the fading properties of dyes you’re interested in using for your processes. Be sure to choose the one that best meets your expectations for how it will fade. Doing the research and testing now can save you from the unexpected color changes down the line.



